The finding suggests that combination therapies aimed at preventing early interactions among these biomarkers, rather than targeting each one individually, may lead to effective treatments, according to the authors.
Beta-amyloid plaque, tau protein neurofibrillary tangles, and neuroinflammation are three known hallmarks associated with Alzheimer's disease. Yet so far, only animal studies have shown how these three biomarkers interact during progression of the disease.
"When these three pathologies are present in the human brain concomitantly, they synergistically interact with each other to determine dementia," wrote first author Dr. Tharick Pascoal, PhD, of the University of Pittsburgh and an international group of colleagues.
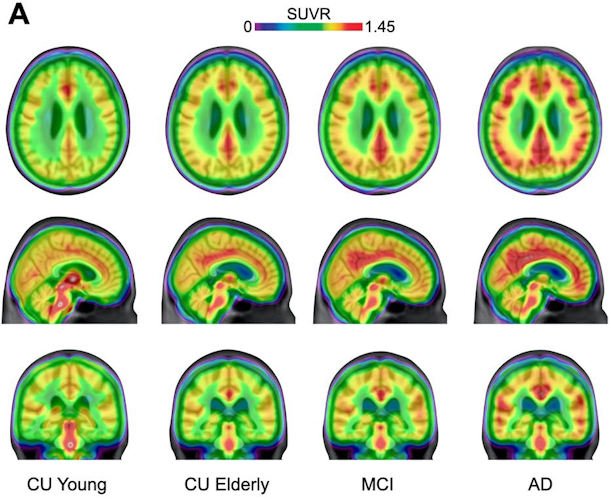
Averaged carbon-11 PBR28 standardized uptake value ratio (SUVR) maps, overlaid on a structural MRI template, suggest a progressively higher uptake in typical Alzheimer's-related regions in the posterior cingulate/precuneus, inferior parietal, and lateral temporal cortices from cognitively unimpaired (CU) young (n = 22) to CU elderly (n = 64), mild cognitive impairment (MCI) (n = 28), and Alzheimer's disease (AD) dementia (n = 16) individuals. Image courtesy of Nature Medicine.
Over the past 20 years, the amyloid cascade hypothesis has guided most research on the brain activity involved in the development of Alzheimer's disease. The cascade hypothesis suggests that beta amyloid is the initial trigger of pathological tau in Alzheimer's disease and precedes symptom onset by decades, while tau is the actual driver of neurodegeneration and cognitive decline.
In addition, compelling experimental evidence from animal studies suggests that neuroinflammation -- the activation of the brain's resident immune cells, called microglial cells -- is also a key element associated with the development of Alzheimer's disease. Microglial cells coexist in the brain alongside beta-amyloid plaques and tau neurofibrillary tangles.
In this study, the researchers hypothesized that the coexistence of widespread beta amyloid, tau, and activated microglia drives dementia symptoms.
Pascoal and colleagues identified 130 individuals with a genetic mutation associated with Alzheimer's disease across the disease spectrum. Patients were divided into four groups: cognitively unimpaired young (22), cognitively unimpaired aged (64), patients with mild cognitive impairment (28), and patients with Alzheimer's disease who had complete cognition (16).
The researchers used a PET radiotracer that specifically targets microglial activity (carbon-11 [C-11] PBR28) and a separate tracer that detected accumulations of beta-amyloid plaque and tau tangles. All patients received a follow-up tau-PET scan after a year.
Importantly, the researchers used a method called Braak staging that essentially allowed them to visually map the progression of dementia by measuring the amounts of tau protein that build up in specific areas of the brain.
Braak stages range from one to six, where stage 1 indicates initial accumulation in the lower brainstem and the olfactory system. Stage 6 indicates tau has fully invaded the neocortex, affecting motor and sensory functions in the brain.
The researchers found "synergistic interaction" between beta-amyloid, tau, and microglial concentrations on PET -- rather than action independently or in groups of two -- was significantly associated with higher rates of dementia.
They found a progressive increase in the percentage of individuals with concomitant beta-amyloid, tau, and microglial activation abnormalities, from 0% in cognitively unimpaired young patients to 4.5% in cognitively aged patients, 27% in those with mild cognitive impairment, and 67% in those with Alzheimer's disease dementia.
"The co-occurrence of [beta-amyloid], tau and microglial activation abnormalities was the strongest predictor of cognitive impairment in our population," the authors wrote.
In addition, the prevalence of microglial activation abnormality alone or together with only beta-amyloid or only tau abnormalities did not differ between clinical diagnostic groups, which further suggests that the presence of both beta amyloid and tau is necessary for the association of microglial activation with cognitive impairment, the researchers stated.
The results have implications for future clinical trials, according to the authors. For one, well-validated brain imaging markers of microglial activation may help assess whether drugs that target the activity are effective, they wrote.
"Our results support that microglial activation is a key element linking the effects of [beta amyloid] to tau spread and ultimately dementia," the researchers concluded.
Copyright © 2021 AuntMinnie.com
"triple" - Google News
August 31, 2021 at 02:05PM
https://ift.tt/3sZnOqS
PET reveals triple threat behind Alzheimer's dementia - AuntMinnie
"triple" - Google News
https://ift.tt/3dc0blF
https://ift.tt/2WoIFUS
Bagikan Berita Ini














0 Response to "PET reveals triple threat behind Alzheimer's dementia - AuntMinnie"
Post a Comment